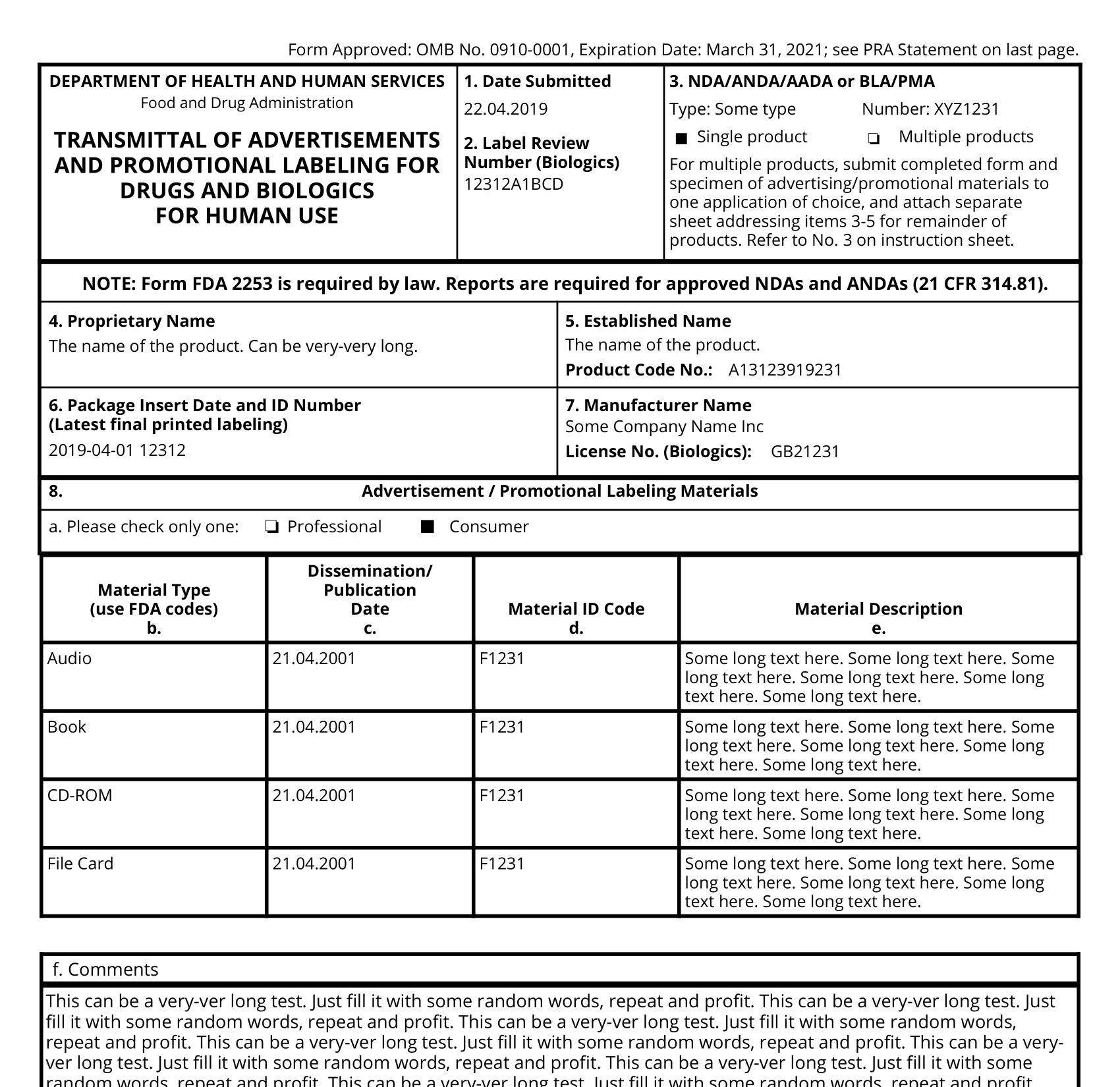
Form FDA 2253 is a document required by the U.S. Food and Drug Administration (FDA) for drug establishments. It is known as the “Transmittal of Adverse Experience Reports” form. The purpose of this form is to provide a standardized format for submitting adverse event reports to the FDA.
1. Compliance with FDA Regulations: Submitting Form FDA 2253 helps drug establishments fulfil regulatory obligations. The FDA requires drug establishments to report any adverse events associated with their products, and using the designated form ensures compliance with these reporting requirements.
2. Streamlined Reporting Process: Form FDA 2253 provides a standardized format for reporting adverse events. This consistency simplifies the reporting process for drug establishments, making it easier to collect and submit the necessary information. It ensures that all relevant details are included uniformly.
3. Enhanced Patient Safety: Adverse event reporting is critical for ensuring patient safety. By promptly reporting adverse events through Form FDA 2253, drug establishments contribute to ongoing drug safety monitoring and evaluation. This information helps the FDA identify potential risks and take appropriate measures to protect public health.
4. Regulatory Oversight and Communication: Submitting adverse event reports allows the FDA to monitor the safety profile of drugs in the market. This information helps regulatory authorities make informed decisions regarding drug approvals, labelling updates, or recalls, if necessary. It also facilitates communication between drug establishments and the FDA, enabling a collaborative approach to ensure drug safety.
5. Continuous Improvement and Risk Mitigation: Adverse event reporting through Form FDA 2253 provides valuable data for drug establishments. By analyzing the reported adverse events, companies can identify patterns, trends, and potential risks associated with their products. This information can be used to implement necessary measures to mitigate risks, improve product safety, and enhance patient outcomes.